The results of the project are available here.
SNAP-1 is an exciting new initiative that we plan to run for the first time in May. It is a particularly important project as we hope that the data collection will be managed predominantly by trainees and will involve hospitals throughout the UK.
The intention is that SNAPs will provide a 'snapshot' evaluation of activity and patient centred outcomes that should be important to both patients and anaesthetists.
SNAP-1 will involve a two day evaluation of patient reported outcome after anaesthesia; specifically, patient satisfaction and patient reported awareness.
We will need trainees in every hospital to play a crucial role in the data collection and those leading this task will be named as collaborators in all future publications.
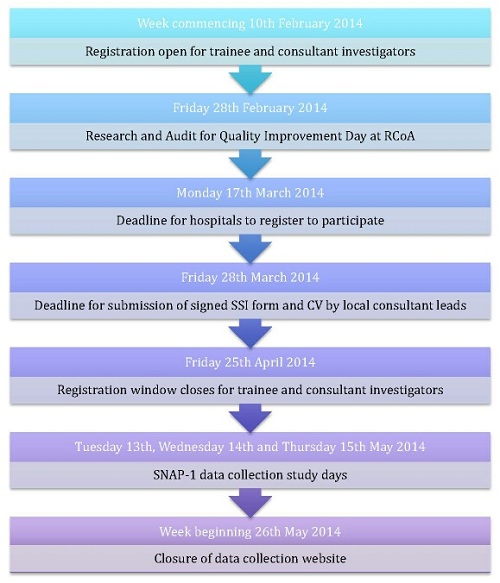
The first Sprint National Anaesthesia Project (SNAP-1) is taking place on Tuesday May 13th and Wednesday May 14th 2014.
Introduction
The Sprint National Anaesthesia Projects (SNAPs) are an exciting new initiative that will run for the first time in Spring 2014. SNAP-1 is being managed by the National Institute of Academic Anaesthesia - Health Services Research Centre (NIAA-HSRC), in conjunction with the UCL/UCLH Surgical Outcomes Research Centre (SOuRCe). It is being funded by an NIAA administered grant provided by the Royal College of Anaesthetists (RCoA).
Background
SNAP-1 aims to provide a 'snapshot' of clinical activity in hospitals throughout the UK over a 2 day period on Tuesday 13th and Wednesday 14th May 2014. It will be a pragmatic evaluation of patient reported outcomes after anaesthesia; specifically patient satisfaction and patient reported awareness.
The intention is that data collection at each site will be managed predominantly by anaesthetic trainees (Local Investigators LI), under the supervision of a Local Lead Investigator (LLI) who may be the Quality Audit and Research Coordinator (QuARC), the departmental audit lead, the clinical director, or another nominated representative.
Ethical considerations
SNAP-1 is a research project, and as such, has undergone ethical review and received approval from the HRA/NRES Committee of the East Midlands. Approval has also been granted for use of a single Site Specific Information (SSI) form. This will reduce the burden on local lead investigators regarding local R&D and ethics sign-offs: all that will be required is a signature of each Local Lead Investigator (LLI) on the SSI form (which will be provided by us) and a one page summary of their CV. SSI forms and guidance on preparing the one-page CV will be sent to LLIs in due course.
Outcome measures
The specific outcome measures used in SNAP-1 will be the validated Patient Satisfaction Questionnaire devised by Bauer et al (see Appendix 1) and the Modified Brice Questionnaire (see Appendix 2) which has been adapted from protocol for the BAG-RECALL clinical trial by Avidan et al.
Objectives
This SNAP aims to establish a national benchmark for patient satisfaction after anaesthesia, which hospitals may then use to evaluate the service that they are delivering to patients and subsequently, if necessary, take action to improve quality.
Secondarily, the intention is to establish an estimate of the incidence of accidental awareness during general anaesthesia in the UK population; this concept of a "Brice day" was part of the activity that was proposed for NAP5.
Inclusion criteria
All adult (≥18 years) patients, undergoing any type of surgery in an operating theatre in an NHS hospital in the UK during the study period.
Exclusion criteria
Patients <18 years old; those who are unable to understand spoken or written English; obstetric patients; patients who are too unwell or confused to be able to complete the questionnaires or those who decline to participate.
How will the project work in my hospital?We anticipate that under the supervision of the Local Lead Investigator, Local Investigators, LIs (who may be anaesthetic trainees, trust grades or consultants) at each hospital will be responsible for the data collection and administration of the two questionnaires to all consenting patients within 24 hours of their operation. The number of LIs required at each site will depend on the size of the hospital and the number of operations performed over the 2-day period. We would suggest that participating LIs are not allocated other clinical responsibilities on the study days but this is obviously a decision that should be made locally.
Each potential participant should be provided with a patient information leaflet (see Appendix 3) on admission to hospital, prior to their surgery, so that they have time to consider the information provided. If they agree to complete and return the questionnaires, this will be taken as implied consent to participate.
The anaesthetist responsible for the care of every patient undergoing surgery on 13 and 14 May will be asked to complete a form with basic patient demographic information (e.g. age, type of surgery, type of anaesthesia) during the operation (see Appendix 4). After completion of the surgery and full recovery from anaesthesia, patients should be approached by LIs or nominated representatives to complete the two paper based questionnaires and told that this should take no more than 10 minutes in total. Patients undergoing day-case procedures will be asked to complete the questionnaires before they are discharged home on the day of their surgery and those who remain as in-patients in hospital should be approached within 24 hours of their operation once they are on a ward. The date and time of completion of the questionnaires will be recorded on the forms themselves. If patients need help understanding the questionnaire or physically filling it in, a Local Investigator should be available to help them do so.
LLIs and/or LIs will then enter the data from the paper questionnaires onto a secure data collection web tool (web link to be provided in due course). Each patient entry on the web tool will generate a unique identifier; Local Investigators will be asked to keep a log of the unique identifiers linked to local hospital identification numbers. No patient identifiable data is to be transferred outside the local hospital environment either electronically or on paper. All paper forms must be destroyed confidentially once uploaded to the data collection web tool.
The log should be held locally until 5 years after study closure at which point it should be destroyed confidentially.
The data collection web tool will be closed for data entry two weeks after the study completion date. After this time, the anonymised responses from hospitals across the UK will be analysed by a team of researchers based at the UCL/UCLH Surgical Outcomes Research Centre in London.
Analysis of Bauer patient satisfaction questionnairesCases will be analysed according to the type of surgery and anaesthetic so as to provide an overall picture of the levels of satisfaction after specific procedures and following different types of anaesthesia. Results will be reported at national level but LLIs will also be able to access a spreadsheet of their own department's data.
Analysis of Brice questionnairesThese questions will be analysed so as to determine the incidence of suspected awareness under anaesthesia nationally. If cases that may describe accidental awareness under general anaesthesia are identified by the researchers, they will refer these reports to an expert panel of assessors (consultant anaesthetists and lay representatives who have been members of the project board of NAP5). If at this stage there is still concern that the patient may have experienced awareness, then the Local Lead Investigators will be contacted and asked to refer to the locally held log in order to identify the patient. LLIs will then be responsible for contacting the patient and following up this potential case of awareness according to their own departmental guidelines.
Further information:
Please refer to the website www.niaa-hsrc.org.uk/SNAPs on which we will be providing updated information regarding all aspects of the project.
Follow @anaesthesiaSNAP on Twitter
Email: [email protected]
The intention is that SNAPs will provide a 'snapshot' evaluation of activity and patient centred outcomes that should be important to both patients and anaesthetists.
SNAP-1 will involve a two day evaluation of patient reported outcome after anaesthesia; specifically, patient satisfaction and patient reported awareness.
We will need trainees in every hospital to play a crucial role in the data collection and those leading this task will be named as collaborators in all future publications.
The first Sprint National Anaesthesia Project (SNAP-1) is taking place on Tuesday May 13th and Wednesday May 14th 2014.
Introduction
The Sprint National Anaesthesia Projects (SNAPs) are an exciting new initiative that will run for the first time in Spring 2014. SNAP-1 is being managed by the National Institute of Academic Anaesthesia - Health Services Research Centre (NIAA-HSRC), in conjunction with the UCL/UCLH Surgical Outcomes Research Centre (SOuRCe). It is being funded by an NIAA administered grant provided by the Royal College of Anaesthetists (RCoA).
Background
SNAP-1 aims to provide a 'snapshot' of clinical activity in hospitals throughout the UK over a 2 day period on Tuesday 13th and Wednesday 14th May 2014. It will be a pragmatic evaluation of patient reported outcomes after anaesthesia; specifically patient satisfaction and patient reported awareness.
The intention is that data collection at each site will be managed predominantly by anaesthetic trainees (Local Investigators LI), under the supervision of a Local Lead Investigator (LLI) who may be the Quality Audit and Research Coordinator (QuARC), the departmental audit lead, the clinical director, or another nominated representative.
Ethical considerations
SNAP-1 is a research project, and as such, has undergone ethical review and received approval from the HRA/NRES Committee of the East Midlands. Approval has also been granted for use of a single Site Specific Information (SSI) form. This will reduce the burden on local lead investigators regarding local R&D and ethics sign-offs: all that will be required is a signature of each Local Lead Investigator (LLI) on the SSI form (which will be provided by us) and a one page summary of their CV. SSI forms and guidance on preparing the one-page CV will be sent to LLIs in due course.
Outcome measures
The specific outcome measures used in SNAP-1 will be the validated Patient Satisfaction Questionnaire devised by Bauer et al (see Appendix 1) and the Modified Brice Questionnaire (see Appendix 2) which has been adapted from protocol for the BAG-RECALL clinical trial by Avidan et al.
Objectives
This SNAP aims to establish a national benchmark for patient satisfaction after anaesthesia, which hospitals may then use to evaluate the service that they are delivering to patients and subsequently, if necessary, take action to improve quality.
Secondarily, the intention is to establish an estimate of the incidence of accidental awareness during general anaesthesia in the UK population; this concept of a "Brice day" was part of the activity that was proposed for NAP5.
Inclusion criteria
All adult (≥18 years) patients, undergoing any type of surgery in an operating theatre in an NHS hospital in the UK during the study period.
Exclusion criteria
Patients <18 years old; those who are unable to understand spoken or written English; obstetric patients; patients who are too unwell or confused to be able to complete the questionnaires or those who decline to participate.
How will the project work in my hospital?We anticipate that under the supervision of the Local Lead Investigator, Local Investigators, LIs (who may be anaesthetic trainees, trust grades or consultants) at each hospital will be responsible for the data collection and administration of the two questionnaires to all consenting patients within 24 hours of their operation. The number of LIs required at each site will depend on the size of the hospital and the number of operations performed over the 2-day period. We would suggest that participating LIs are not allocated other clinical responsibilities on the study days but this is obviously a decision that should be made locally.
Each potential participant should be provided with a patient information leaflet (see Appendix 3) on admission to hospital, prior to their surgery, so that they have time to consider the information provided. If they agree to complete and return the questionnaires, this will be taken as implied consent to participate.
The anaesthetist responsible for the care of every patient undergoing surgery on 13 and 14 May will be asked to complete a form with basic patient demographic information (e.g. age, type of surgery, type of anaesthesia) during the operation (see Appendix 4). After completion of the surgery and full recovery from anaesthesia, patients should be approached by LIs or nominated representatives to complete the two paper based questionnaires and told that this should take no more than 10 minutes in total. Patients undergoing day-case procedures will be asked to complete the questionnaires before they are discharged home on the day of their surgery and those who remain as in-patients in hospital should be approached within 24 hours of their operation once they are on a ward. The date and time of completion of the questionnaires will be recorded on the forms themselves. If patients need help understanding the questionnaire or physically filling it in, a Local Investigator should be available to help them do so.
LLIs and/or LIs will then enter the data from the paper questionnaires onto a secure data collection web tool (web link to be provided in due course). Each patient entry on the web tool will generate a unique identifier; Local Investigators will be asked to keep a log of the unique identifiers linked to local hospital identification numbers. No patient identifiable data is to be transferred outside the local hospital environment either electronically or on paper. All paper forms must be destroyed confidentially once uploaded to the data collection web tool.
The log should be held locally until 5 years after study closure at which point it should be destroyed confidentially.
The data collection web tool will be closed for data entry two weeks after the study completion date. After this time, the anonymised responses from hospitals across the UK will be analysed by a team of researchers based at the UCL/UCLH Surgical Outcomes Research Centre in London.
Analysis of Bauer patient satisfaction questionnairesCases will be analysed according to the type of surgery and anaesthetic so as to provide an overall picture of the levels of satisfaction after specific procedures and following different types of anaesthesia. Results will be reported at national level but LLIs will also be able to access a spreadsheet of their own department's data.
Analysis of Brice questionnairesThese questions will be analysed so as to determine the incidence of suspected awareness under anaesthesia nationally. If cases that may describe accidental awareness under general anaesthesia are identified by the researchers, they will refer these reports to an expert panel of assessors (consultant anaesthetists and lay representatives who have been members of the project board of NAP5). If at this stage there is still concern that the patient may have experienced awareness, then the Local Lead Investigators will be contacted and asked to refer to the locally held log in order to identify the patient. LLIs will then be responsible for contacting the patient and following up this potential case of awareness according to their own departmental guidelines.
Further information:
Please refer to the website www.niaa-hsrc.org.uk/SNAPs on which we will be providing updated information regarding all aspects of the project.
Follow @anaesthesiaSNAP on Twitter
Email: [email protected]
To register:
http://www.niaa-hsrc.org.uk/SNAP_Registration
http://www.niaa-hsrc.org.uk/SNAP_Registration